The U.S. Food and Drug Administration has approved a new preventative treatment for migraines in adults.
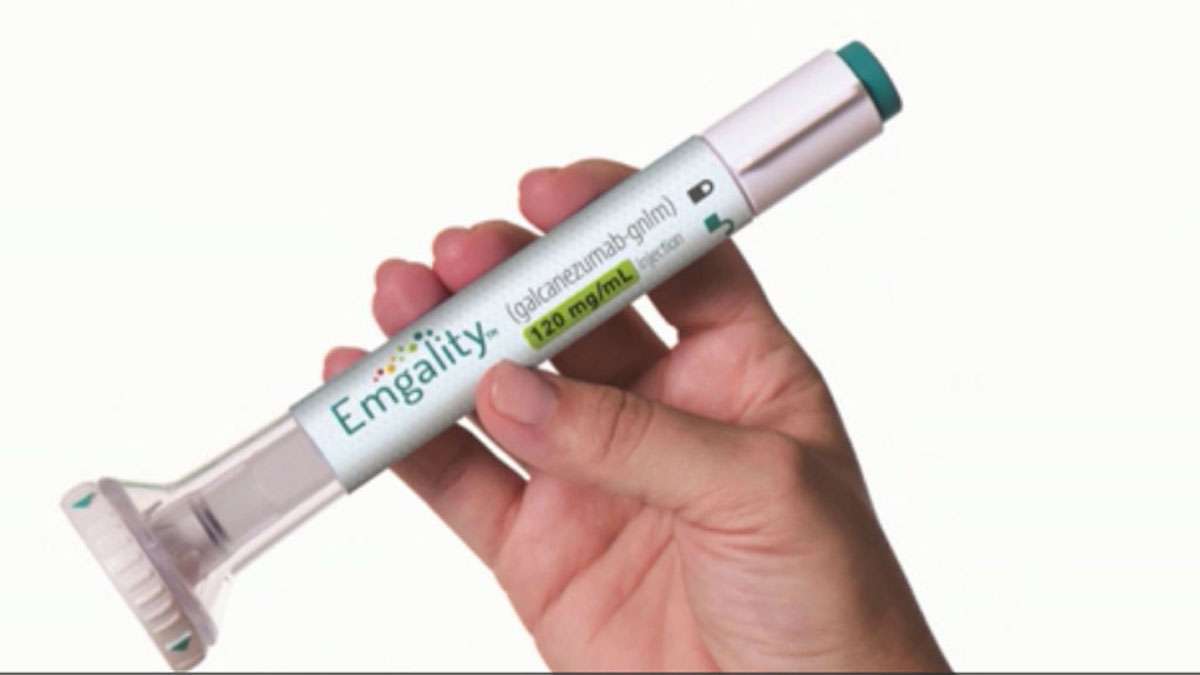
The treatment, which is called Emgality, is a once-monthly, self-administered injection that was developed by global pharmaceutical company Eli Lilly and Company.
"Despite the devastating impact of migraine, only about 10 percent of people living with the disease are currently taking a preventive treatment," said Christi Shaw, president of Lilly Bio-Medicines. "With this approval, we are thrilled to offer a preventive treatment option to adults living with this disease."
Migraine is a disabling, neurologic disease that affects more than 30 million American adults. According to the Medical Expenditures Panel Survey, the total unadjusted cost associated with migraine in the U.S. is estimated to be as high as $56 billion annually, yet migraine remains "under-recognized and under-treated".
But now, hope may be on the horizon for migraine sufferers. According to several double-blind, placebo-controlled studies, the Emgality injections had no major side effects and they cut chronic and episodic migraines by as much as 50% – which is being hailed as "a huge deal" by physicians.
Emgality will be available to patients shortly after approval. Patients with commercial insurance may also be able to receive up to 12 months of the treatment for free as part of Lilly's patient support program.
(WATCH the emotional CBS coverage below) – Photo by Eli Lilly and Company
Be Sure And Share The Good News With Your Fellow Migraine Sufferers On Social Media









Be the first to comment