Taking Ketamine and Looking at Smiling Faces is a Therapy That Could Help End Depression, Shows Clinical Trial
Being given ketamine and then looking at smiling faces could help end depression, according to a new scientific study.
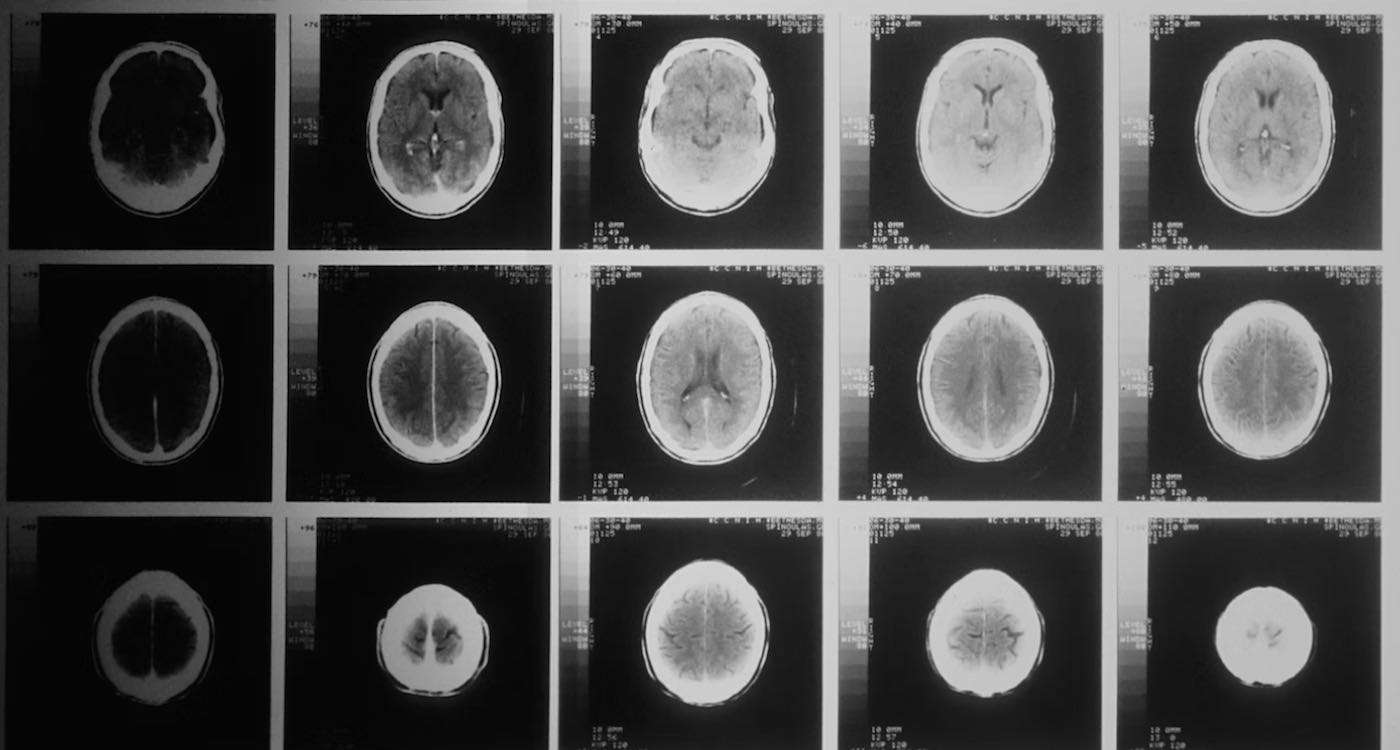
American biotech company Biogen announced the results of a phase 3 clinical trial that showed a new Alzheimer's drug slowed the rate of cognitive decline for early onset patients by 27%.
The Alzheimer's Association (AA) called the robust study of lecanemab, which is a monoclonal antibody designed to clear clumps of amyloid protein from the brain, "the most encouraging results in clinical trials treating the underlying causes of Alzheimer's to date."
The CEO at Eisai-the Japanese pharmaceutical company partnering with Biogen-claims the results of the lecanemab study, named Clarity AD, "proves the amyloid hypothesis, in which the abnormal accumulation of Aβ in the brain is one of the main causes of Alzheimer's disease."
Eisai believes these findings will create new horizons in the diagnosis and treatment of Alzheimer's disease as well as further activate innovation for new treatment options.
The study enrolled 1,800 patients with early stage Alzheimer's, with half receiving placebo and half receiving twice-weekly infusions of the lecanemab. Results showed the drug did reduce toxic amyloid plaque in the brain and slow patients' memory decline, while improving their ability to perform day-to-day tasks.
The full analysis of the trial has yet to be released.
Alzheimer's advocates and researchers look forward to learning more about the data at a meeting in November, including participant safety and the percentage of patients who experienced any brain swelling.
"If those data are consistent with what we saw today regarding efficacy and safety, we strongly support FDA approval and full [Medicare] coverage," added the AA in a statement.
"Today's announcement gives patients and their families hope that lecanemab, if approved, can potentially slow the progression of Alzheimer's disease, and provide a clinically meaningful impact on cognition and function," said Michel Vounatsos, Chief Executive Officer at Biogen.
The U.S. Food and Drug Administration (FDA) has agreed that the results of the 18-month trial can serve as the confirmatory study to verify the clinical benefit of lecanemab, setting a date of January 6 to announce its decision.
However, researchers say that amyloid is only one component of Alzheimer's disease-and some are calling the benefits measured in this trial "small".
Amyloid is "associated with the problem, but it isn't 'the' problem", a neurobiologist at the University of Texas and a sceptic of the amyloid hypothesis told NATURE. "If you modulate it, of course you can have some small benefit."
But others are chiming a much more hopeful note.
"This is a historic moment for dementia research, as this is the first phase 3 trial of an Alzheimer's drug in a generation to successfully slow cognitive decline," said Dr Susan Kohlhaas, the director of research at Alzheimer's Research UK.
"Many people feel Alzheimer's is an inevitable part of aging. This spells it out: if you intervene early you can make an impact on how people progress."
HOPEFUL? Then, Share the Results on Social Media to Spread the Optimism
Be the first to comment