Researchers announced that they have essentially stopped the progression of amyotrophic lateral sclerosis (ALS), or Lou Gehrig's disease, for nearly two years, allowing the mice to approach their normal lifespan.
Scientists at Oregon State University say the findings are some of the most compelling ever produced in the search for a therapy to help sufferers of ALS, a debilitating and fatal disease.
"We are shocked at how well this treatment can stop the progression of ALS," said Joseph Beckman, lead author on this study and professor of biochemistry and biophysics in the College of Science at Oregon State University, and principal investigator at OSU's Linus Pauling Institute.
WANT MORE BREAKTHROUGH NEWS? …GET OUR GOOD NEWS APP —> Download FREE for Android and iOS
In decades of work, no treatment has been discovered for ALS that can do anything but prolong human survival less than a month. The mouse model used in this study is one that scientists believe may closely resemble the human reaction to this treatment.
It's not yet known if humans will have the same response, but researchers are moving as quickly as possible toward human clinical trials, testing first for safety and then efficacy of the new approach, which consists of a compound called copper-ATSM.
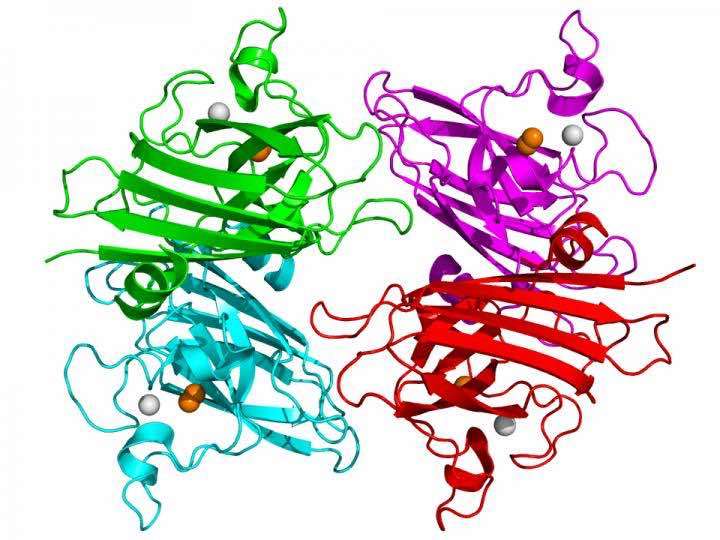
ALS is known to be caused by the death and deterioration of motor neurons in the spinal cord, which in turn has been linked to mutations in copper, zinc superoxide dismutase.
Copper-ATSM is a known compound that helps deliver copper specifically to cells with damaged mitochondria, and reaches the spinal cord where it's needed to treat ALS. This compound has low toxicity, easily penetrates the blood-brain barrier, is already used in human medicine at much lower doses for some purposes, and is well tolerated in laboratory animals at far higher levels. Any copper not needed after use of copper-ATSM is quickly flushed out of the body.
Experts caution, however, that this approach is not as simple as taking a nutritional supplement of copper, which can be toxic at even moderate doses. Such supplements would be of no value to people with ALS, they said.
Using the new treatment, researchers were able to stop the progression of ALS in one type of transgenic mouse model, which ordinarily would die within two weeks without treatment. Some of these mice have survived for more than 650 days, 500 days longer than any previous research has been able to achieve.
In some experiments, the treatment was begun, and then withheld. In this circumstance the mice began to show ALS symptoms within two months after treatment was stopped, and would die within another month. But if treatment was resumed, the mice gained weight, progression of the disease once again was stopped, and the mice lived another 6-12 months.
"We have a solid understanding of why the treatment works in the mice, and we predict it should work in both familial and possibly sporadic human patients," Beckman said. "But we won't know until we try."
Familial ALS patients are those with more of a family history of the disease, while sporadic patients reflect the larger general population. In humans who develop ALS, the average time from onset to death is only three to four years.
The treatment is based on bringing copper into specific cells in the spinal cord and the mitochondria weakened by copper deficiency. Copper is a metal that helps to stabilize SOD, an antioxidant protein whose proper function is essential to life. But when it lacks its metal co-factors, SOD can "unfold" and become toxic, leading to the death of motor neurons. (See photo courtesy of Oregon State University.)
There's some evidence that this approach, which works in part by improving mitochondrial function, may also have value in Parkinson's disease and other conditions, researchers said. Research is progressing on those topics as well.
The treatment is unlikely to allow significant recovery from neuronal loss already caused by ALS, the scientists said, but could slow further disease progression when started after diagnosis. It could also potentially treat carriers of SOD mutant genes that cause ALS.
The new findings were reported in Neurobiology of Disease. This work has been supported by the U.S. National Institutes of Health, the Amyotrophic Lateral Sclerosis Association, which received a huge bump in funding throughout the Ice Bucket Challenge last year, the Australian National Health and Medical Research Association, the Department of Defense Congressionally Directed Medical Research Program, and gifts by Michael Camillo and Burgess and Elizabeth Jamieson to the Linus Pauling Institute.
(Photo released by Oregon State University)
SHARE the Good News…







Be the first to comment