Bee Buzzes Could Help Determine How to Save Their Decreasing Population
This soon-to-be smartphone app could help thousands of bee enthusiasts monitor their local honeybee population and save them from decline.
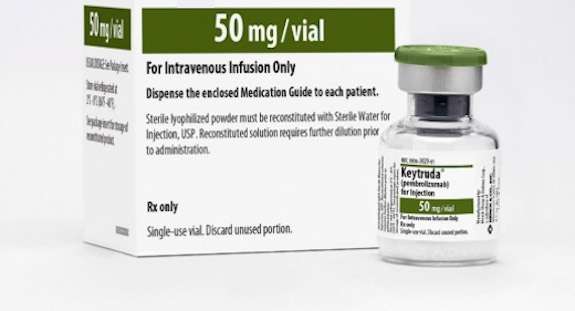
This groundbreaking new drug for cancer treatment was immediately approved by the FDA in light of its revolutionary effects on tumors across the body.
The drug, known as pembrolizumab (brand name Keytruda), is the first cancer treatment that can be used against 11 different kinds of cancers no matter their location; whether in the bone, colon, pancreas, brain, or lung, as long as the tumor has certain bio-markers.
Many of these advanced types of cancer work by shielding themselves from the proteins that the immune system uses to detect and fight diseases. Pembrolizumab, however, is an example of an immunotherapy drug called a PD-1 blocker. These drugs detect tumors by their genetic code so that they can reveal them to the immune system, which can then work against them accordingly.
While the specific genetic code that pembrolizumab targets isn't very common, the drug could still save over 60,000 Americans from rare cancers every year.
The researchers at Johns Hopkins Medicine first stumbled upon the genetic treasure chest years ago when a clinical trial of over a dozen patients were treated with an immunotherapy drug – none of them showed any kind of response, except for one, whose cancer disappeared altogether.
When researchers looked at the tumor's genetics, they found a specific mutation that matched several other types of cancers. This led them to developing the drug that targeted the mutation: pembrolizumab.
"This was the eureka moment that led us to develop this clinical trial," says Bert Vogelstein, co-director of the Ludwig Center at the Johns Hopkins Kimmel Cancer Center and the Genetics Program of the Bloomberg-Kimmel Institute.
In light of the drug's efficiency, the FDA quickly approved the use of pembrolizumab in May.
It is now marketed by pharmaceutical company Merck & Co., and will cost approximately $100,000 per year.
Click To Share The Groundbreaking News With Your Friends (Photo by Mercke)
Be the first to comment