New Design for Solid-State Hydrogen Fuel Cell Significantly Reduces Charging Times and Improves Safety
Researchers at the University of Technology Sydney significantly improved hydrogen fuel cell charging times for safer solid-state storage.
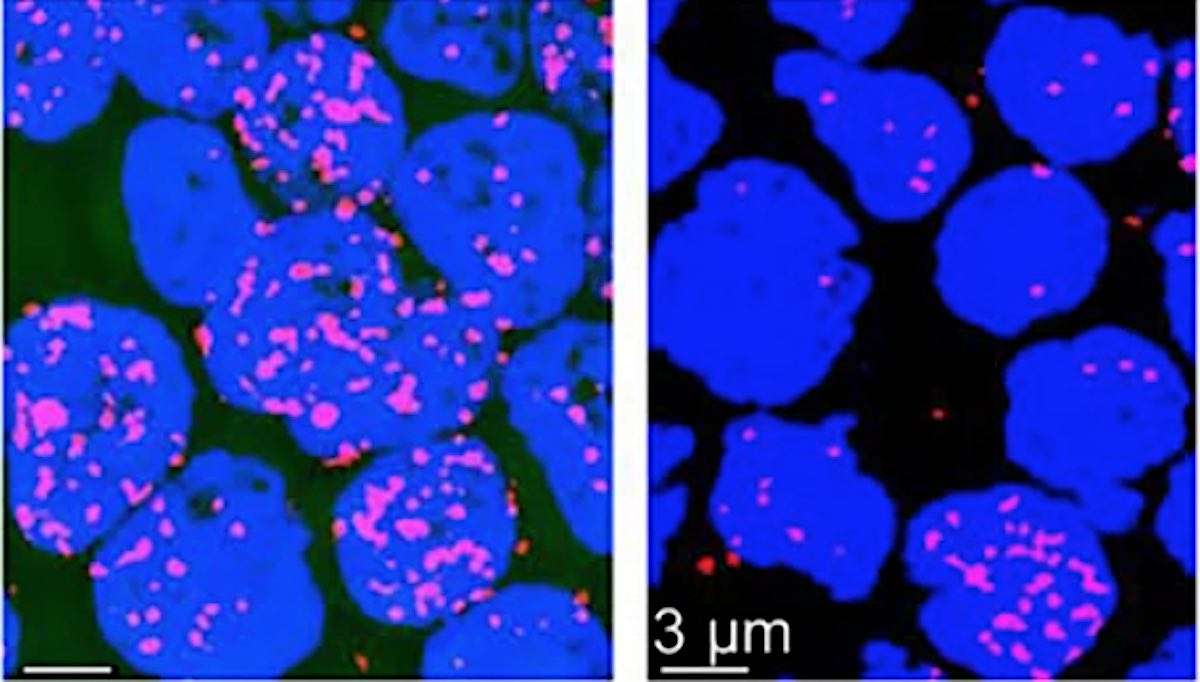
DNA replication and repair happens thousands of times a day in the human body and most of the time, people don't notice when things go wrong thanks to the work of Replication protein A (RPA), the 'guardian of the genome.'
Scientists previously believed this protein 'hero' responsible for repairing damaged DNA in human cells worked alone, but a new study by Penn State College of Medicine researchers showed that RPA works with an ally called the WAS protein (WASp) to 'save the day' and prevent potential cancers from developing.
The researchers uncovered these findings after observing patients with Wiskott-Aldrich syndrome (WAS), which not only suppresses immune system function but, in some cases, causes cancer. They realized that this genetic disorder is caused by a deficiency of WASp.
Dr. Yatin Vyas, professor and chair of the Department of Pediatrics at Penn State College of Medicine and pediatrician-in-chief at Penn State Health Children's Hospital, conducted prior research that revealed that WASp functions within an apparatus that is designed to prevent cancer formation.
As a result, some cancer patients had tumor cells with a WASp gene mutation. These observations led him to hypothesize that WASp might play a direct role in DNA damage repair.
"WAS is very rare-less than 10 out of every 1 million boys has the condition," said Vyas. "Knowing that children with WAS were developing cancers and also observing WASp mutations in tumor cells of cancer patients, we decided to investigate whether WASp plays a role in DNA replication and repair."
The researchers conducted protein-protein binding experiments with purified human WASp and RPA and discovered that WASp forms a complex with RPA. Further tests revealed that WASp 'directs' RPA to the site where single DNA strands are broken and need to be repaired.
According to Vyas, without the complex, DNA repair happens by secondary mechanisms, which can lead to cancer. This novel function of WASp is conserved through evolution, from yeast to humans. The results of the study were published in Nature Communications.
Vyas said it is possible that gene therapy or stem cell therapy could restore WASp function and may prevent further tumor growth and spread. He also mentioned the possibility of using WASp dysfunction as a biomarker for identifying patients at risk for autoimmune diseases and cancers.
In the future, Vyas and colleagues will continue to study how their observations about this RPA-WASp complex formation can be applied to treating cancer patients.
"This complex we've discovered plays a critical role in preventing the development of cancers during DNA replication," Vyas told Penn State Health News. "Translating this discovery from bench to bedside could mean that someday we have another tool for predicting and treating cancers and autoimmune diseases."
SPREAD the Good News About Cancer Research on Social Media…
Be the first to comment