Stem-Cell Based 'Cure' for Type-1 Diabetes Draws Nearer, With FDA Trials Launched
Creating insulin-producing pancreas cells safely and at 80%, a new method using 3-dimensional petri dishes draws type-1 diabetes cure nearer.
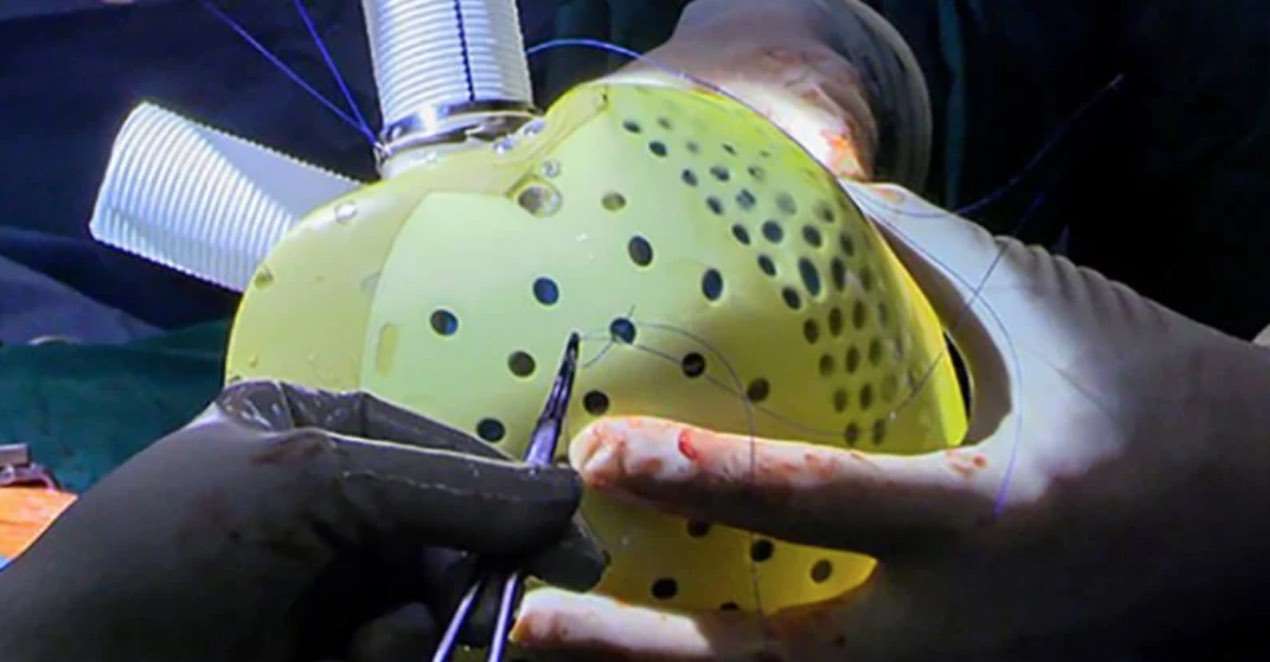
Surgeons at Duke University successfully implanted a new-generation artificial heart in a man with heart failure—a first for any hospital in North America.
The artificial heart was developed by a French company, CARMAT and has been approved for use and sale in Europe.
Last year, the company received U.S. FDA approval to begin studies and enroll 10 patients with end-stage biventricular heart failure—people who are suffering on the waiting list for a heart donor—and offer a life-saving bridge before transplant.
"We are encouraged that our patient is doing so well after the procedure Monday," said Dr. Carmelo Milano, a transplant surgeon and the principal investigator of the device study at Duke. "As we evaluate this device, we are both excited and hopeful that patients who otherwise have few to no options could have a lifeline."
The North Carolina patient, Matthew Moore, is just 39-years-old and was referred to Duke in June after a sudden, unexpected diagnosis of heart failure. Moore and his wife, Rachel, recently adopted their two-year-old foster son, Marshall, and arrived at Duke expecting only to undergo heart bypass surgery.
As Moore's condition quickly deteriorated, however, traditional options, including transplant, became too risky. Meanwhile, Duke was among just three transplant centers in the United States selected to join the device study, and the procedure team received specialized training to prepare for the implant surgery.
Matthew's wife, a nurse, said, "Both Matthew and I are so grateful that we've been provided an opportunity to participate in something that has the potential to have an impact on so many lives. We are just taking it day-by-day and hope everything continues to progress well."
The artificial heart device called the ‘Aeson', is an implantable prosthetic that includes biological valves derived from bovine tissue and operates on an external power supply.
If the device receives FDA approval, it would provide hope for transplant patients whose hearts require assistance to pump blood through both chambers. Current technology—notably a left-ventricular assist device (LVAD)—supports just one chamber.
"Because of the shortages of donor hearts, many patients die while waiting for a heart transplant," said Schroder, a transplant surgeon who led the implant procedure. "We are hopeful for new options to help these patients, many like Mr. Moore who have devastating disease and cannot otherwise be considered for a transplant."
WATCH a CBS report…
SHARE the Heartwarming Innovation on Social Media…
Be the first to comment