Psychedelic Drugs May Be Able to Treat Brain Injuries, Stimulating New Neurons to Replace Impaired Ones
The idea is that psychedelics could keep the critical windows open long enough to recover far more function than would otherwise be the case.
For the first time, genetically modified pig kidneys provided "life-sustaining kidney function" during the course of a planned seven-day clinical study—a first step in addressing the critical crisis worldwide of kidney donor organ shortage.
The University of Alabama's pre-clinical human study at Birmingham also advances the science and promise of xenotransplantation as a therapy to potentially cure end-stage kidney disease—just as a human-to-human transplants can.
"It has been truly extraordinary to see the first-ever preclinical demonstration that appropriately modified pig kidneys can provide normal, life-sustaining kidney function in a human safely and be achieved using a standard immunosuppression regimen," said UAB transplant surgeon scientist Jayme Locke, M.D., director of UAB's Comprehensive Transplant Institute and lead author of the paper.
"The kidneys functioned remarkably over the course of this seven-day study," she said. "We were able to gather additional safety and scientific information critical to our efforts to seek FDA clearance of a Phase I clinical trial in living humans and hopefully add a new, desperately needed solution to address an organ shortage crisis responsible for tens of thousands of preventable deaths each year."
The peer-reviewed findings published last month in JAMA Surgery describes the pioneering pre-clinical human research performed on a recipient experiencing brain death by the Locke and Heersink School of Medicine team. It comes 19 months after last year's groundbreaking peer reviewed UAB xenotransplant study in which genetically modified pig kidneys were successfully transplanted into a recipient after brain death.
The pre-clinical human brain death model developed at UAB can evaluate the safety and feasibility of pig-to-human kidney xenografts, or transplants, without risk to a living human. It is named for transplant pioneer Jim Parsons, an organ donor whose family generously donated his body to advance xenotransplant kidney research, like the latest patient did.
A Critical Need
Kidney disease kills more people each year than breast or prostate cancer, while more than 90,000 people are on the transplant waiting list. More than 800,000 Americans are living with kidney failure and 240 Americans on dialysis die every day. The wait for a deceased donor kidney can be as long as five to 10 years, and almost 5,000 people per year die waiting for a kidney transplant.
Groundbreaking Study Details
The 52-year-old study subject for this research lived with hypertension and stage 2 chronic kidney disease, which affects more than one in seven U.S. adults, or an estimated 37 million Americans. As part of this study, the subject had both of his native kidneys removed and dialysis stopped, followed by a crossmatch-compatible xenotransplant with two 10 gene-edited pig kidneys, or UKidney.
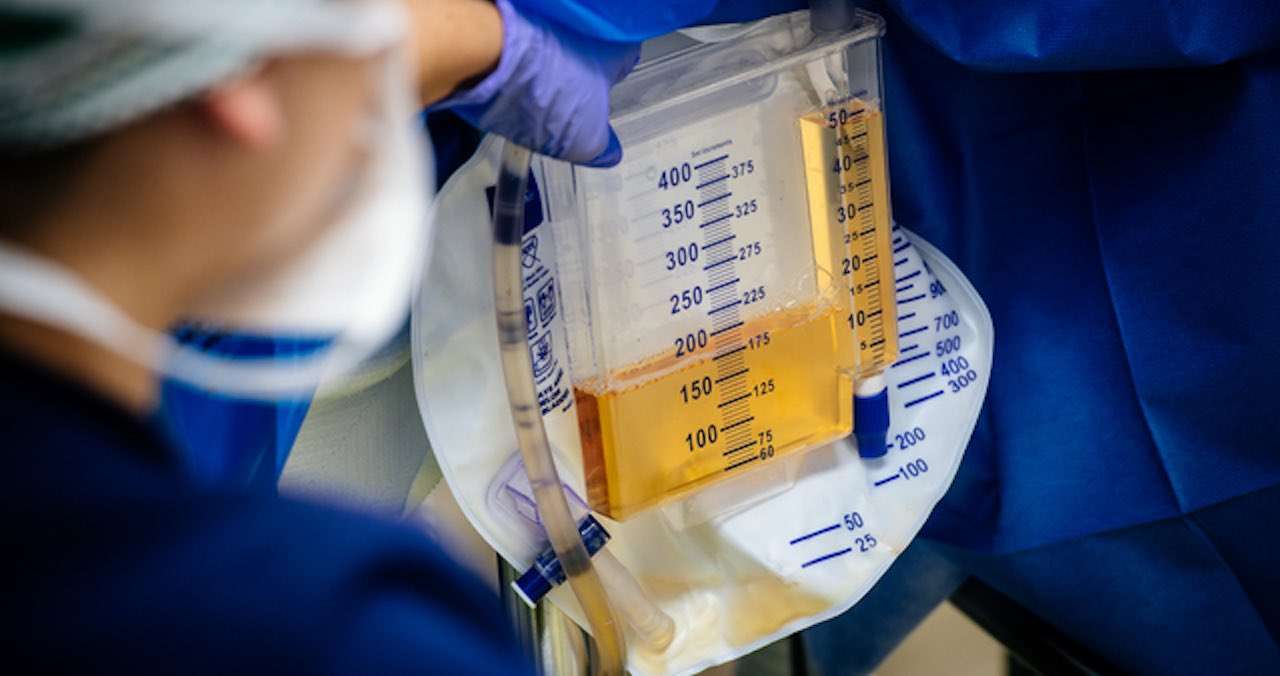
The transplanted pig kidneys made urine within four minutes of re-perfusion and produced more than 37 liters of urine in the first 24 hours. The pig kidneys continued to function as they would in a living human for the entirety of the seven-day study. Also, the kidneys were still viable at the time the study was concluded.
"In the first 24 hours these kidneys made over 37 liters of urine," said Dr. Locke. "It was really a remarkable thing to see."
The pig kidneys were serially biopsied throughout the course of the study. Biopsies showed normal histology by light microscopy without evidence of any destruction of red blood cells, low platelets or organ damage due to the formation of microscopic blood clots in capillaries or small arteries.
Gene editing in pigs to reduce immune rejection has made organ transplants from pigs to humans possible. The natural lifespan of a pig is 30 years, they are easily bred, and they have organs of similar size to humans. Genetically modified pig kidneys have been extensively tested in non-human primates, and the addition of UAB's preclinical human research model—the Parsons Model—now provides important information about the safety and efficacy of kidneys in human transplant recipients.
HELP the 800,000 Americans With Kidney Disease by SHARING on Social Media…
Be the first to comment